ASTMH Advocacy
Advocating for tropical medicine/global health research and development is a key component of ASTMH’s mission as a professional scientific society. As one of our top priorities, alongside our government relations experts, the society aims at informing Members of Congress, lobbying and advocating for the tropical medicine and global health of the NIH, CDC, Department of Defense and USAID.
ASTMH is a member of the Global Health Technologies Coalition (GHTC), a coalition of more than 25 nonprofit organizations advancing policies to accelerate the creation of new drugs, vaccines, diagnostics and other health tools that bring healthy lives within reach for all people.
Society Takes Action After U.S. Approves Use of Antimalarials for COVID-19 Treatment
In March 2020, the Society responded to the FDA acting under its authority through the Emergency Use Authorization to provide assistance in a public health emergency to provide Emergency Use Authorization of the antimalarial and anti-inflammatory 4-aminoquinolines chloroquine and hydroxychloroquine for the treatment or prevention of COVID-19.
President Joel G. Breman, MD, DTPH, FIDSA, FASTMH, and CEO Karen A. Goraleski sent a signed letter to FDA Commissioner Stephen M. Hahn, MD, FASTRO, on March 31 expressing concern over the side effects from chloroquine and hydroxychloroquine, and the loss of drug availability for many patients with systemic lupus erythematosis or rheumatoid arthritis, for which the drug is clearly indicated.
Read our letter to the FDA here
ASTMH Applauds CDC for Making IV Artesunate Available for Severe Malaria...
 CDC's annoucement that it will make intravenous artesunate available for the treatment of severe malaria once quinidine is no longer available beginning April 1, 2019, was applauded by the Society.
CDC's annoucement that it will make intravenous artesunate available for the treatment of severe malaria once quinidine is no longer available beginning April 1, 2019, was applauded by the Society.
“Artesunate is the best treatment for severe malaria,” said ASTMH President Chandy C. John, MD, MS, FASTMH, “and making it the first-line therapy is a big advance for malaria treatment in the United States. We thank the CDC, the Walter Reed Army Institute of Research and the U.S. Army Medical Materiel Development Activity for their efforts to make this treatment available. We’re looking forward to their update on how clinicians will be able to rapidly obtain artesunate for their patients with severe malaria, and what treatment they should provide while waiting to receive artesunate. The answers to these important questions will be critical in ensuring the best care for patients with severe malaria."
Read the ASTMH statement supporting the CDC's announcement to make intravenous artesunate available for the treatment of severe malaria
...And Urges the FDA to Approve Artesunate for General Use in the U.S.
On June 17, 2019, ASTMH sent a letter to the FDA expressing concern about the lack of an FDA approved drug for the treatment of severe malaria in the United States. "The CDC recommends treatment with artesunate, the international standard for treatment of severe malaria, but the only way to obtain this drug in the US is through a request to the CDC... ASTMH is strongly advocating for FDA approval of artesunate as standard therapy for severe malaria in the United States, and for general availability of this important drug. Numerous international clinical trials have demonstrated unequivocally that it is the current gold standard for therapy of severe malaria," stated the letter, signed by ASTMH President Chandy C. John, MD, MS, FASTMH, and CEO Karen A. Goraleski.
Review the letter to the FDA urging the approval of artensuate for general use in the U.S.
'Vaccines Save Lives: What is driving Prentable Disease Outbreaks?'
 U.S. Senate Committee on Health, Education, Labor and Pensions (HELP) hearing on preventing disease outbreaks from March 5. View the video here to see the excellent questions and inquiries from Democrats and Republicans. Download the testimonies by clicking here and scrolling down.
U.S. Senate Committee on Health, Education, Labor and Pensions (HELP) hearing on preventing disease outbreaks from March 5. View the video here to see the excellent questions and inquiries from Democrats and Republicans. Download the testimonies by clicking here and scrolling down.
CDC Vital Signs: Trends in Reported Vectorborne Disease Cases — United States and Territories, 2004–2016
Between 2004 and 2016, more than 640,000 cases of vector-borne disease like dengue, Zika, Lyme, or plague were reported in the U.S., and 9 new germs spread by bites from infected mosquitoes and ticks were discovered or introduced. Better control of mosquitoes and ticks is needed to protect people from these costly and deadly diseases.
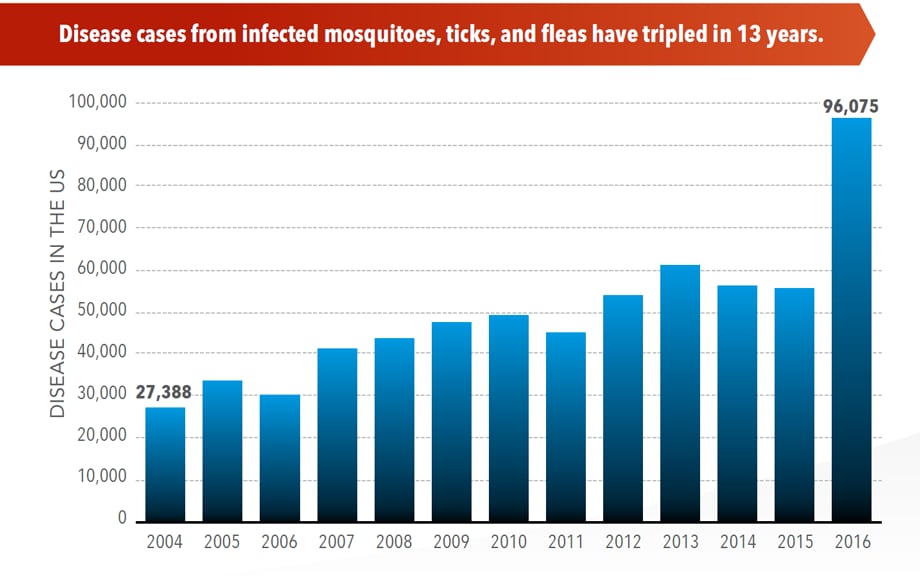
View the CDC's Vital Signs page, with statistics and infographics
Read the CDC Morbidity and Mortality Weekly Report
Global Health R&D Matters
 Global health R&D matters globally and benefits you locally. Find out how with this interactive U.S. map showing investments in global health R&D paying off in economic and health dividends in your state.
Global health R&D matters globally and benefits you locally. Find out how with this interactive U.S. map showing investments in global health R&D paying off in economic and health dividends in your state.
Created by the Global Health Technologies Coalition (GHTC), a coalition of more than 25 nonprofit organizations advancing policies to accelerate the creation of new drugs, vaccines, diagnostics and other health tools that bring healthy lives within reach for all people. ASTMH is a member of the GHTC and CEO Karen A. Goraleski is a board member.
CDC Global Health Security
Learn more about the economic impact of CDC’s global health security portfolio. Two recent CDC-authored articles in the journal Health Security analyze the risks the American export economy faces from global outbreaks:
Relevance of Global Health Security to the U.S. Export Economy takes a look at how public health emergencies in other countries can influence U.S. jobs and exports.
Impact of Hypothetical Infectious Disease Outbreak on U.S. Exports and Export-Based Jobs examines what could happen to the U.S. economy if an epidemic were to strike a key region, such as Southeast Asia.
Additionally, Is the U.S. Export Economy at Risk from Global Infectious Outbreaks? is related CDC press release that includes a quote from Acting CDC Director Dr. Anne Schuchat.
GHTC Fact Sheets
 ASTMH is a member of the Global Health Technologies Coalition, a coalition of more than 25 nonprofit organizations advancing policies to accelerate the creation of new drugs, vaccines, diagnostics and other health tools that bring healthy lives within reach for all people. CEO Karen A. Goraleski is a board member.
ASTMH is a member of the Global Health Technologies Coalition, a coalition of more than 25 nonprofit organizations advancing policies to accelerate the creation of new drugs, vaccines, diagnostics and other health tools that bring healthy lives within reach for all people. CEO Karen A. Goraleski is a board member.
In October 2017, GHTC released a series of fact sheets examining the importance of global health research and development, and the contributions of U.S. government agencies to advancing global health R&D:
Who we are: Global Health Technologies Coalition
Describes GHTC’s mission and membership. View the PDF.
Why does global health R&D matter?
Explains why global health R&D is vital in achieving a safer, healthier world. View the PDF.
What has global health R&D achieved?
Outlines the historical progress achieved through global health R&D and the impact of US leadership. View the PDF.
Why is global health R&D a smart investment for America?
Describes the health, security, and economic returns from investing in global health R&D. View the PDF.
Global health R&D at USAID
Describes the US Agency for International Development (USAID)’s role in global health R&D. View the PDF.
Global health R&D at NIH
Describes the National Institutes of Health (NIH)’s role in advancing basic research to solve global health challenges. View the PDF.
Global health R&D at CDC
Describes the role of the US Centers for Disease Control and Prevention (CDC) in global health R&D to protect individuals at home and abroad from infectious diseases. View the PDF.
Global health R&D at FDA
Describes the Food and Drug Administration (FDA)’s role in regulating and supporting life-saving global health solutions. View the PDF.
Global health R&D at DoD
Describes the Department of Defense (DoD)’s support for global health R&D to protect US service members abroad and support national security. View the PDF.
Global health R&D at BARDA
Describes the growing role of the Biomedical Advanced Research and Development Authority (BARDA) in global health R&D. View the PDF.
Tips to Use When Meeting with Elected Officials
The 2016 Annual Meeting included the session, "The Washington, DC Primer: Advocating for R&D Funding – The Who, What, Where, Why, & How," which focused on advocacy efforts with presenters Past President Stephen Higgs, PhD, FRES, FASTMH, Jodie Curtis of the District Policy Group and ASTMH CEO Karen A. Goraleski.
Curtis, of the District Policy Group, provided background on the then-current state of funding of neglected disease research and the role of U.S. government agencies such as NIH, CDC, USAID and DoD. The presentation, “Where Does Global Health Funding Come From?,” also included practical tips for researchers to use when meeting with an elected official to convey their concerns.
View a copy of Jodie’s slide deck here and feel free to use it for reaching out to your elected officials to emphasize the importance of the US government’s global health R&D funding.
Presidents urge Congress to take action on Zika


About ASTMH Advocacy
 Advocating for investments (U.S. federal funding) in tropical medicine/global health research and development (R&D) is a key component of ASTMH’s mission as a professional scientific society. The Society has as one of its top priorities the need to inform Members of Congress and their staff on the value and importance of robust funding for tropical medicine/global health programs. With strategic input from the society’s government relations experts, the Board identifies the specific areas of focus for lobbying and advocacy efforts, such as tropical medicine/global health programs within NIH, CDC, Department of Defense and USAID.
Advocating for investments (U.S. federal funding) in tropical medicine/global health research and development (R&D) is a key component of ASTMH’s mission as a professional scientific society. The Society has as one of its top priorities the need to inform Members of Congress and their staff on the value and importance of robust funding for tropical medicine/global health programs. With strategic input from the society’s government relations experts, the Board identifies the specific areas of focus for lobbying and advocacy efforts, such as tropical medicine/global health programs within NIH, CDC, Department of Defense and USAID.
ASTMH engages in targeted lobbying and advocacy initiatives throughout the year including participating in Capitol Hill meetings, submitting expert testimony to Congress, and collaborating with partner organizations on shared appropriation and policy goals. ASTMH members offer a unique ability to magnify the Society’s efforts by contacting their own members of Congress’ offices through emails and visits. A strong U.S. investment in global health research and development saves lives and saves money, and advocacy by the scientific community is essential to protecting those investments.
Register for legislative updates by clicking here.
FY 2016 US Federal Funding Tropical Medicine/Global Health
| Agency |
FY 2015 Final |
FY 2016 Final |
% Change from
FY 2015 Final |
| NIH |
$30 Billion |
$32 Billion |
+ 6.6% |
| NIAID |
$4.3 Billion |
$4.6 Billion |
+ 6.2% |
| Fogarty |
$67.7 Million |
$70.4 Million |
+ 3.9% |
| CDC |
$6.8 Billion |
$7.2 Billion |
+ 4.8% |
| CDC Parasitic Diseases and Malaria |
$24.3 Million |
$24.5 Million |
+ 0.5% |
| CDC Emerging and Zoonotic Infectious |
$404.9 Million |
$527.8 Million |
+ 30.3% |
| USAID Global Health/Child Survival |
$2.7 Billion |
$2.8 Billion |
+ 1.7% |
| USAID NTDs |
$100 Million |
$100 Million |
- |
| USAID Malaria |
$669.5 Million |
$674 Million |
+ 0.6% |
Why should you – as an individual – advocate?
- Members of Congress need and want to hear from you, their constituents.
- If Members don’t hear from individual members of the science community, they are hearing from someone else and someone else’s issues will get prioritized.
- Lobbyists can be a good resource, but “real scientists” have the expertise, stories and experiences that carry the most meaning to policymakers and staff.
- Members value the opinion of constituents (you vote them in and vote them out) and you can shape the advocacy process by engaging with policymakers.
Malaria no more and ASTMH reception on Capitol Hill 2016
ASTMH Leadership May 2015 Hill Day Prep (PPT)
April 9, 2015, Letter Re Federal Employee Travel Restrictions to Attend Scientific Conferences
ASTMH Testimony to House Labor Health Human Services Appropriations 2016
ASTMH Testimony to House in Support of Stronger Department of Defense Tropical Medicine 2016
Advocacy Pointers
- Talk about the work you do and address why it matters to the U.S. (remember U.S. taxpayers are paying for this so Congress also wants to be sure they hear what the U.S. gets out of this investment). Be brief!
- Members of Congress need a personal story – the work you do, the lives saved, the jobs created, the economic impact, the security impact, etc.
- In this economic climate, hearing about job creation via research dollars is high on the list of what policymakers and staff are interested to hearing about.
- Do a little bit of homework and familiarize yourself with your Member’s position on various policy issues by looking at their website.
- Have a clear “ask” when visiting your Member (e.g, “I’d like you to visit our lab and see what we are doing on xyz”) and be sure to provide local examples (e.g., “Our latest grant brought $xx to our state and created xx jobs; here’s why studying malaria at Xxx Midwestern University matters to the people who live in our state”).
- Always follow up with your Member after a meeting by sending a short thank you letter/email.
The American Society of Tropical Medicine and Hygiene (ASTMH) — the nation’s leading professional organization for tropical medicine — represents approximately 4,000 researchers and clinicians engaged in the battle against infectious and tropical disease in the United States and internationally. ASTMH promotes global health through research and education to prevent and control tropical diseases.